A2M - Mark Nuijten
Welcome on our website
A2M, partner of Minerva Network, provides international consulting services in healthcare environment. Bridging our International Know-how and Experience in Market Access&Reimbursement, Health Economics, Valuation, Pricing, we will able to offer a taylor-made solution to your specific needs, which will increase the Value of your Medical Innovation
- Positive and rapid reimbursement and market access
- Maximum sales at premium price
- Optimal cash flows
- Maximum economic value of innovation
The dossiers by a2m successfully contributed to positive reimbursement decisions by health authorities in many countries, including United Kingdom, Sweden, Belgium and The Netherlands.
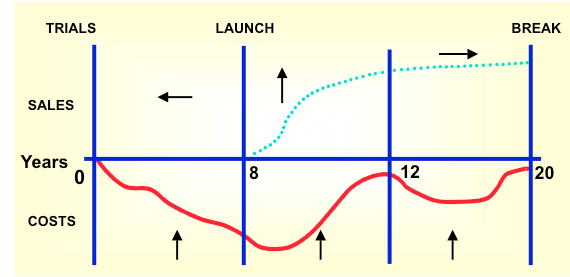
The Critical Success factor is the Strategic Value Scan, which will:
- Optimalise cash flows across the entire life cycle
- Maximise patients’ quality of life and shareholder value.
The figure shows the relation between the Strategic Value Scan and the key drivers of market access.
Example: The cost-effectiveness, BIA and pricing potential depend on the positioning of the new product and the expected comparators for each population.

Business Cases
- Reimbursement dossier for a new biological in MS in Belgium and The Netherlands
- Valuation of a phase I drug in oncology for portfolio investment decisions and licensing negotiations
- Development of an economic message for nutritional supplements for payers in Germany
- Single technology assessment dossier for NICE and SMC in the UK for new class antidepressant
- Valuation of a start up biotech company with three compounds in development in oncology, orphan disease and cardiovascular diseases
- Budget impact analysis for pain medication in Belgium
- Global core cost-effectiveness model development in chronic kidney disease for rolling out to European markets, Canada and Australia
- European pricing strategy and valuation scan for immunotherapy
- Pan European market access and reimbursement for innovative medication in pain management
- Patient access scheme development for disease management program in obesity
- Model adaptation of global core model in Parkinson’s disease to The Netherlands
- Price negotiations In Netherlands for so-called “”expensive”drug in the lock (”sluis”)
- Corporate strategy for market access portfolio of nutritionals
- Future positioning of new ant diabetic treatment combined with stratified medicine in European markt
- Organisation of an International Advisory Board for development of clinical guidelines for forthcoming in breast cancer.
- Development of Core value Dossier for Phase II antibiotic treatment
- Model adaptation for new MS drug in global markets outside Europe and US: Japan, Russia, South Korea, Brazil, Argentina, and Mexico